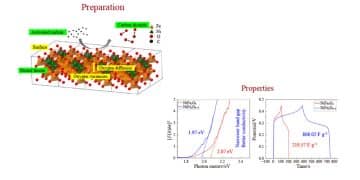
Oxygen vacancies engineering is widely acknowledged as a potent strategy for augmenting the electrochemical performance of metal oxides in the realm of supercapacitors.
Researchers from Jinan School of Materials Science and Engineering, Shandong University, China published their study Activated carbon induced oxygen vacancies-engineered nickel ferrite with enhanced conductivity for supercapacitor application.
In recent research by Prof. Jianqiang Bi’s team, NiFe2O4−δ, characterized by a profusion of oxygen vacancies, was successfully synthesized through a subsequent heat treatment process within an activated carbon bed, building upon the foundation of the hydrothermal-synthesized NiFe2O4.
The meticulous treatment yielded the NiFe2O4−δ, which exhibited superior conductivity and a remarkable 3.7-fold increase in capacitance compared to its NiFe2O4 counterpart. This observed enhancement in electrochemical properties underscores the pivotal role played by oxygen vacancies in optimizing the performance of metal oxides.
The results of their study strongly support the notion that the deliberate introduction of oxygen vacancies holds substantial promise for advancing the electrochemical properties of metal oxides, thereby positioning them as promising materials for supercapacitor electrodes. Their work was published in the journal Frontiers of Chemical Science and Engineering.
This newfound understanding opens avenues for potential applications in the field of energy storage, showcasing the significant impact of oxygen vacancy engineering on the development of high-performance supercapacitors.
Abstract
NiFe2O4 is a kind of bimetallic oxide possessing excellent theoretical capacity and application prospect in the field of supercapacitors. Whereas, due to the inherent poor conductivity of metal oxides, the performance of NiFe2O4 is not ideal in practice. Oxygen vacancies can not only enhance the conductivities of NiFe2O4 but also provide better adsorption of OH, which is beneficial to the electrochemical performances. Hence, oxygen vacancies engineered NiFe2O4 (NiFe2O4‒δ) is obtained through a two-step method, including a hydrothermal reaction and a further heat treatment in activated carbon bed.
Results of electron paramagnetic resonance spectra indicate that more oxygen vacancies exist in the treated NiFe2O4‒δ than the original one. UV-Vis diffuse reflectance spectra prove that the treated NiFe2O4‒δ owns better conductivity than the original NiFe2O4. As for the electrochemical performances, the treated NiFe2O4‒δ performs a high specific capacitance of 808.02 F∙g‒1 at 1 A∙g‒1. Moreover, the asymmetric supercapacitor of NiFe2O4‒δ//active carbon displays a high energy density of 17.7 Wh∙kg‒1 at the power density of 375 W∙kg‒1. This work gives an effective way to improve the conductivity of metal oxides, which is beneficial to the application of metal oxides in supercapacitors.
Reference link to the article:
Xicheng Gao, Jianqiang Bi, Linjie Meng, Lulin Xie, Chen Liu. Activated carbon induced oxygen vacancies-engineered nickel ferrite with enhanced conductivity for supercapacitor application. Front. Chem. Sci. Eng., 2023, 17(12): 2088‒2100 https://doi.org/10.1007/s11705-023-2352-6
Read the original post at Advancing Electrochemical Properties of Metal Oxides for Supercapacitor Electrodes