This article is an excerpt from ESA SPCD 2022 paper entitled “Novel Graphene Material for High Energy Storage Supercapacitors” written by Tomáš Zedníček, EPCI, Czech Republic and Michal Otyepka, Aristeidis Bakandritsos, Veronika Šedajová, CATRIN, Palacký University Olomouc, Czech Republic that was presented during the 4th ESA SPCD conference at ESA ESTEC, The Netherlands 11-14th October 2022. Published under ESA SPCD organisation committee permission.
Abstract
Supercapacitors have attracted great interest due to their high energy density, practically unlimited charging/discharging number of cycles and sustainability. However, compared to battery technologies their energy densities still remain lower. In the last decade, supercapacitors with an energy content of ∼110 Wh/L at a power of ∼1 kW/L were developed by leveraging the open framework structure of graphene-related architectures. The research described in this paper report a novel method of high energy density graphene materials prepared based on fluorographene.
The best performing highly nitrogen doped (~16%) graphene (GN3), with diamond-like bonds and an ultra-high mass density of 2.8 g cm−3, is an excellent host for the ions, delivering unprecedented energy densities of up to 200 WhL-1 at a power of 2.6 kW L-1 and 143 Wh L-1 at 52 kW L-1. These findings open a route to materials whose properties may enable a transformative improvement in the performance of supercapacitor components. The paper explains the technology background and features of this high-energy graphene-based supercapacitors, their challenges, and potentials for future applications.
Introduction
The pursuit of materials for efficient electricity storage is one of the key challenges for today’s science. The ever-increasing mobility and number of electronic devices, along with ongoing efforts to reduce fossil fuel consumption, will continue to spur global demand for affordable, reliable and sustainable energy. The goal is not only to continuously increase battery capacity but also to look for other efficient non-lithium-based ways of storing electricity. Carbon-based supercapacitors are beginning to be an attractive alternative for energy storage, mainly due to their safety, long-life and extraordinary ability to sustain millions of charging cycles without losing capacity.
Current research in SC is primarily focused on highly porous, high surface area activated carbons (i.e., 2000 m2g-1), since the energy storage mechanism is based on the formation of the electrostatic double layer at the interface between the electrode material and the electrolyte. Such carbons have a very low density (c.a. 0.3-0.5 gcm-3), leading to a large empty space in the electrode which is then flooded with electrolyte. Thus, the electrode not only consumes large volume but also gets heavier, while the electrolyte-filled space does not add to capacitance. Therefore, the highest so far obtained volumetric energy densities (parameter of critical importance for practical applications) of carbon-based SCs have been obtained from densely packed graphene architectures, ranging between 80 to 100 Wh L-1. These values are ~5 times higher than commercial high performance active carbons and they can thus compete with lead acid and metal hydride batteries. Theoretically, though, graphene could potentially reach 300 WhL-1.
Development of High-Energy Graphene Electrode Supercapacitors
EDLCs currently mostly use as electrodes Activated Carbon or Carbon Nanotubes (CND). The potential benefits of graphene electrodes vs carbon includes:
- More cost-efficient, enormous surface area (2630 m2 g-1) and higher power density.
- 10x more conductive.
- Theoretical max capacitance of 2D graphene is 550 F g-1 (~200 F g-1 achieved in practice).
- Easy team up with various other nanomaterials, prominently carbon nanotubes (CNTs), to create low cost, lightweight and high-performance SCs.
We have considered several design strategies how to boost graphene-based electrode material charge storage potential such as:
- Increase the surface area (microporous 3D structure).
- Reduce restacking.
- Increase the packing density and conductivity.
- Accomplish defect control.
- Functionalization and hybridization of electrode materials.
Based on the above strategies we set the aim of the research into two directions how to boost the capacitance and energy density of graphene-based electrode materials:
- #1 Mounting of longer functional groups perpendicular to the graphene surface to further enhance capacitance.
- #2 Doping with heteroatoms such as N-doping, that increases the electronic conductivity and improves the solid-electrolyte interface, allowing solvated ions to better infiltrate the pores of the electrode – increasing both electrostatic and pseudo-capacitance mechanism.
The proposed way to meet the objectives was to use covalent functionalization of graphene and N-doping, with fluorographene proposed as the precursor for functionalization. This process has been proved in previous work2,4 as a low cost, ideal to create high-density matching material with graphene-like monolayer.
The graphite fluoride material is an industrial lubricant available on the market in tonnes, which increases its potential commercial availability and smooth transition into mass manufacturing. At the same time, the material and related processes are environmentally friendly and upon selecting the right electrolyte it yields into sustainable, environmentally friendly, supercapacitor design.
Energy Density Boosted by Functionalized Group #1
The target of the activity was to assemble redox-active reversible materials (such as transition metal oxides – MnO2, iron oxides or 2D MXenes) as a long functionalizing group perpendicular to the 2D graphene structure in order to create creating nano-pores and nano-channels boosting the charge storage mechanisms.
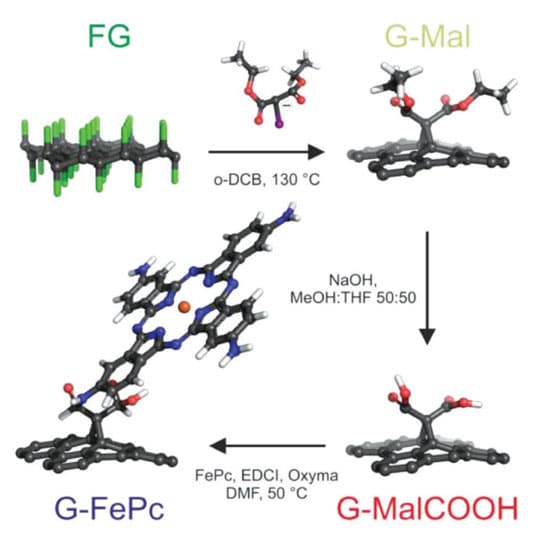
Metal iron tetraaminophthalocyanine G-FePc was selected as the best candidate matching the fluorographene manufacturing route using Bingel-Hirsch reaction2. (Figure 1.)
Energy Density Boosted by Nitrogen Doping #2
The nitrogen doping of graphene via mild and low energy processes to afford homogeneous product composition and topology with high nitrogen content (>10 at. %) remains a challenge of contemporary 2D materials chemistry.
We reported a carbon-based electrode material, GN3, with an unprecedented density of 2.8 g cm−3 and an N2 sorption-based surface area of 128 m2 g−1 that can host ions even more efficiently than carbon materials with surface areas exceeding 2000 m2 g−1. GN3, which is prepared by reacting graphite fluoride with sodium azide, has tetrahedral (sp3) C–C bonds, which were identified in the same region as the C–C bonds in diamond. However, it retains a 2-D structure with a very high content of aromatic (and thus conductive) regions, together with nitrogen superdoping in the vacancies and holes of the aromatic lattice. (Figure 2.)
This new class of carbon-based materials comprising nitrogen doped graphene with diamond-like tetrahedral bonds for high energy density supercapacitor electrodes. The cells with electrodes made from these materials offer ground-breaking energy storage capability at very high charging/discharging rates. The discovery of this class of materials will spur intense research on other high-density conductive carbon materials with different functionalities, with the aim of further increasing the competitiveness of supercapacitors in the portable energy storage landscape.
Experimental
Samples of functionalized graphene (Figure 3.) and N-doped graphene GN3 (Figure 4.) were prepared using fluorographene as the precursor. Details of chemistry and processes – see scientific publication “Nitrogen doped graphene with diamond-like bonds achieves unprecedented energy density at high power in a symmetric sustainable supercapacitor“4.
Electrodes prepared from active electrode powder were inserted into the capacitor contact holder and characterized using a measurement jig. Cycle lifetime at room temperature was also evaluated – see Figure 5.
Results
Using this new approach, we achieved several important milestones for graphene-based supercapacitors as summarized below and in Ragone plot on Figure 6.
G-FePc Funcionalized Graphene Supercapacitors:
- SC cell voltage 1V with an aqueous liquid
- Up to 1000 F g-1 energy density at 59 Wh kg-1 and power density of 0.3 kW kg-1
- Limiting factor: G-FePc high cost
2D N-Doped Graphene:
- SC cell voltage with ionic liquid: 3.7V (high cell voltage compared to conventional SCs)
- Up to 128 F g-1 at ~55 Wh kg-1 and 2 kW kg-1 corresponds to 357 F cm-3 at 170 Wh L-1 and 5 kW L-1 (lower gravimetric ED compared to covalently functionalized graphene). Importantly, this represents a record volumetric energy density, best in class ever reported for SCs (comparable to batteries).
- At higher power density (required for SCs), up to 300 F cm-3 140 Wh L-1 and 52 kW L-1 (record power density, significantly higher when compared to conventional SCs).
- 100% capacitance retention after 10 000 charge-discharge cycles
GN3 material, nitrogen super doped graphene with diamond-like layer bonds, achieved record energy density in a range of conventional batteries while power density is close to capacitors capability.
Next Steps
Mass Production Ready High Energy Graphene-Based Supercapacitors
The next step will be to build prototypes of supercapacitors in co-operation with international partners. We will focus on optimizing the properties of GN3 material and move to pilot production of the graphene-based electrode supercapacitors.
The aim is to increase the energy density of supercapacitors beyond 50 Wh L-1, which will allow their wide use in electric vehicles and as battery supports in devices that need to be supplied with large amounts of energy in a very short time.
For the commercial application, the next step includes the preparation of SC prototypes by our industrial partners in a flat pack and wound packaging form to demonstrate its capabilities in the most common SC forms on the market today.
Use of Fluorographene Process to Enhance LiS Batteries
The fluorographene process has also the potential to enhance the electrode system in LiS batteries.
Sulphur represents a low‑cost, lighter weight, sustainable, and high theoretical capacity cathode material for lithium–sulphur batteries. However, the shuttling‑effect of the formed lithium polysulfides, as well as their low conductivity, compromise the electrochemical performance of lithium–sulphur cells.
Li-S batteries advantage:
- 3-5x higher energy density ~ 2,500 Wh/kg
- Nickel and cobalt free solution (conflict/resource issues free)
- No heavy metals / 3x lighter compared to Li-Ion
- No / suppressed thermal runaway failure
- Cheaper: ~200 tons of sulfur equals to ~1 ton of cobalt
- Lower carbon footprint, RoHS friendly
- 3x Faster charging
- No extra pressure needed (unlike solid states)
- Vigorous 100% charge/discharge depth rate
To tackle this challenge, a so far unexplored cathode, composed from sulfur covalently bonded directly on graphene is developed. This is achieved by leveraging the nucleophilicity of polysulfide chains, which react readily with the electrophilic centers in fluorographene, as experimental and theoretical data unveil. The reaction leads to the formation of carbon–sulfur covalent bonds and a particularly high sulfur content of 80 mass%.
The first results of Li-S batteries with enhanced electrodes by fluorographene process keep the capacity at ≈700 mAh g−1 after 100 cycles at 0.1 C, at ≈644 mAh g−1 after 250 cycles at 0.2 C and ≈470 mAh g−1 even after 500 cycles that present promising results for further optimization and development.
Summary and Conclusion
The presented research led to the development of new high energy graphene-based electrode material and preparation processes for a new generation of supercapacitors (SC) as highly efficient, high-energy density and sustainable storage components.
The nitrogen-doped GN3 graphene material that has been developed is proving to be very promising for use in supercapacitors. The material has a higher density than graphite, which, combined with the great ability to host ions from the electrolyte, leads to very high volumetric energy density, i.e., the amount of energy stored per unit of volume of the material. This is significantly higher than for all carbon- or graphene-based supercapacitor materials described so far, which can bring a breakthrough improvement in the performance of supercapacitors.
The GN3 based structure supercapacitors demonstrated herein have reached energy density as high as 200Wh L-1 and power density up to 50 kW L-1 that presenting a record achievement reported at the time of the research. Laboratory made supercapacitors confirmed excellent stability of the material, no/low wear out during charge / discharge cycle and long lifetime of the system. Further optimization of the GN3 material for prototyping and pre-production is under process to prepare for mass production of the novel graphene SCs in an industrial scale.
Achievement summary:
- New generation of graphene-based supercapacitor electrode material with high mass density
- Record-high volumetric energy and power densities
- Patent application filed
- Scalable synthesis ready for pre-production
Acknowledgement
The research has been supported by:
- European Union’s Horizon 2020: ERC project No. 683024
- European Union’s ERC Grant: ERC PoC 899245
- European Regional Development Fund: Operational Programme Research, Development and Education CZ.02.1.01/0.0/0.0/16_019/0000754
- Ministry of Education, Youth and Sports of the Czech Republic: LO1305, CZ.1.05/2.1.00/19.0377, Research Infrastructure NanoEnviCz: project No. LM2015073
- Internal Student Grant Agency of the Palacký University in Olomouc, Czech Republic (IGA_PrF_2021_031, IGA_PrF_2022_019).
IP Protection
- EP 20173178.3 – Title: Nitrogen doped graphene and use thereof
- PCT/CZ2021/050016 – Follow-up Application
- EP 20199904.2 – Title: Sulfur-functionalized graphene, and use thereof as Li-S battery cathode
- PCT/CZ2021/050045 – Follow-up Application
References
- [1] H. Li, Y. Tao, X. Zheng, J. Luo, F. Kang, H.-M. Cheng, Q.-H. Yang, Energy Environ. Sci. 2016, 9, 3135.
- [2] A. Bakandritsos et al. Adv. Funct. Mater. 2018, 28 (29), 1801111
- [3] IDTechEx report https://www.idtechex.com/en/research-report/supercapacitors-applications-players-markets-2020-2040/661
- [4] VŠedajová et al.; Nitrogen doped graphene with diamond-like bonds achieves unprecedented energy density at high power in a symmetric sustainable supercapacitor; Energy Environ. Sci. 2022, 15 (2), 740-748, doi 10.1039/d1ee02234b
- [5] Y. Xu, Z. Lin, X. Zhong, X. Huang, N. O. Weiss, Y. Huang, X. Duan, Nat. Commun. 2014, 5, 5554.
- [6] S. Murali, N. Quarles, L. L. Zhang, J. R. Potts, Z. Tan, Y. Lu, Y. Zhu, R. S. Ruoff, Nano Energy 2013, 2, 764.
- [7] H. Jin, X. Feng, J. Li, M. Li, Y. Xia, Y. Yuan, C. Yang, B. Dai, Z. Lin, J. Wang, J. Lu, S. Wang, Angew. Chem. Int. Ed. 2019, 58, 2397.
- [8] X. Yang, C. Cheng, Y. Wang, L. Qiu, D. Li, Science 2013, 341, 534.
- [9] H. Li, Y. Tao, X. Zheng, J. Luo, F. Kang, H.-M. Cheng, Q.-H. Yang, Energy Environ. Sci. 2016, 9, 3135.
- [10] M. Acerce, D. Voiry, M. Chhowalla, Nat. Nanotechnol. 2015, 10, 313.
- [11] Z. Li, S. Gadipelli, H. Li, C. A. Howard, D. J. L. Brett, P. R. Shearing, Z. Guo, I. P. Parkin, F. Li, Nat. Energy 2020, 5, 160.